 Shannon Murphy joined the program as a first-year graduate student January 1, 2018 and is expected to graduate in 2022. Her major is chemistry and she completed her undergraduate degree in analytical chemistry at Albion College, Michigan. Her research topic is using capillary electrophoresis-mass spectrometry to analyze small molecule metabolites (neurotransmitters and amino acids) in biological samples during neurological development.
Shannon Murphy joined the program as a first-year graduate student January 1, 2018 and is expected to graduate in 2022. Her major is chemistry and she completed her undergraduate degree in analytical chemistry at Albion College, Michigan. Her research topic is using capillary electrophoresis-mass spectrometry to analyze small molecule metabolites (neurotransmitters and amino acids) in biological samples during neurological development.
Murphy has presented her research at multiple venues, including the Pittcon Analytical Conference (2020), Society for Neuroscience Conference (October 2019), American Society for Mass Spectrometry Conference on Mass Spectrometry and Allied Topics (June 2019), the Turkey Run Analytical Chemistry Conference (November 2018), and the MBM Retreat (October 2018 + September 2019). She is completing lab rotations under the advisement of Jonathan Sweedler and Martha Gillette.
Murphy has participated in other trainee activities such as the Kickoff meeting, Summer Journal Club readings/discussion meetings, Frontiers in Miniature Brain Machinery lectures, and the MBM Retreat. She received awards for Best Lightning Talk at the 2019 and 2020 MBM Retreats.

Her community outreach activities through MBM include assisting with the MBM booths at the Beckman Open House in March 2019 and the Urbana Farmer’s Market in June 2019. Other outreach activities include Brain Awareness Day at the Orpheum Children’s Science Museum (April 2018), Women’s Chemist Committee Bonding with Chemistry Girls Day Camp (Summer 2018 + Summer 2019), Women’s Chemist Committee First Year Student Mentor (August 2018 – Present) and the Girls’ Adventures in Mathematics, Engineering, and Science (GAMES) Camp (June 2019). She is also mentoring an undergraduate student in Jonathan Sweedler’s lab (2019-2020).
As one of our first cohort of trainees, Murphy is in her sixth semester of the Special Topics in MBM course and has completed coursework in integrative neuroscience, evolutionary neuroscience, advanced analytical chemistry, concepts in chemical biology, scientific ethics, advanced professional development for chemists, and preparing graduate fellowships. In addition, she has attended workshops in science communication led by Sandra Tsing Loh and Jennifer Follis at Beckman Institute for Advanced Science and Technology (2018 & 2019), and participated in the EBICS Engineering Cellular Systems Summer School (August 2018).
Research Highlights
Project 1: Measuring neurotransmitters during rodent central nervous system and spinal cord organoid development in collaboration with Amanda Weiss from the M. Gillette lab
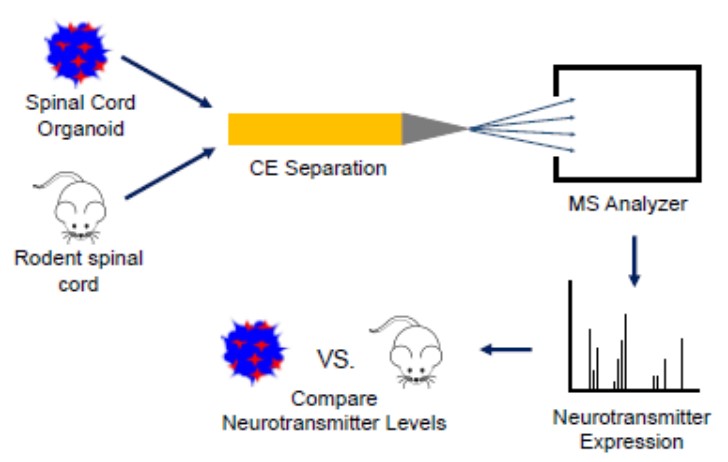
Spinal cord organoids are 3D aggregates of motor neurons and glia that are cultured from stem cells in vitro which have emerged as important models for studying disease and development. Studies have shown that spinal cord organoids demonstrate anatomical features of the central nervous system (CNS); however, their ability to recapitulate the cell-to-cell signaling molecule complement of the CNS has not fully been investigated. This work aims to understand the extent to which spinal cord organoids neurochemically model rodent spinal cord explants.
We are accomplishing this by analyzing the neurotransmitter expression in spinal cord organoids and mouse spinal cord explants during development using capillary electrophoresis-electrospray ionization-mass spectrometry (CE-ESI-MS). I have quantified the neurotransmitters acetylcholine, γ-aminobutyric acid (GABA), glutamic acid, and aspartic acid in postnatal day 0 (P0) and postnatal day 7 (P7) mouse spinal cord explants. Preliminary data show a possible concentration increase of acetylcholine, GABA, aspartic acid, and glutamic acid in juvenile (P7) vs. newborn (P0) mouse spinal cord explants.
This potential trend is expected because rodents gain the ability to perform various reflexes between the P0 and P7 timepoints. More biological replicates are needed to see a significant difference in neurotransmitters between the two timepoints. In collaboration with the Gillette lab, I have also successfully detected the neurotransmitter glutamic acid in 55-day-old spinal cord organoid samples. Current work is focused on developing a centrifugation method to separate spinal cord organoids from Matrigel in order to analyze the organoids using CE-MS and on quantifying neurotransmitters in 16-day-old spinal cord organoids.
Future work will involve quantifying neurotransmitters in 23-26-day-old spinal cord organoids and comparing results to data from the spinal cord extracts at similar developmental timepoints to each other to determine the extent to which spinal cord organoids chemically model the CNS.
Project 2: Measuring monoamine neurotransmitters in planarians to determine their role in germ cell development in collaboration with Professor Phil Newmark and Umair Khan from the University of Wisconsin-Madison
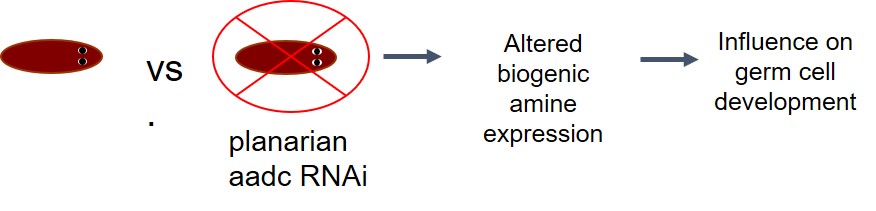
The objective of using capillary electrophoresis-mass spectrometry (CE-ESI-MS) to measure monoamine neurotransmitters in planarians is to determine their role in germ cell development. This then can provide insight and guide future studies of germ cell development in mammals. Work was done to optimize a CE-ESI-MS method for detection of the monoamine neurotransmitters tyramine, tryptamine, dopamine, octopamine, serotonin, norepinephrine, epinephrine, L-Dopa, synephrine, melatonin, N-Acetylserotonin, and 5-Hydroxytryptophan.
Figures of merit were demonstrated through reproducible injections measured by having a percent relative standard deviation (%RSD) of 9-21% for peak intensity and 9-16% for peak area for most of the compounds of interest. Synephrine had a higher %RSD of 28% for peak area and the neurotransmitters melatonin, N-Acetylserotonin, and 5-Hydroxytryptophan were not able to be reproducibly detected.
With a method developed that could reproducibly detect most compounds of interest, the next steps were focused on analyzing monoamine neurotransmitters in planarian extracts. This proved to be difficult at first because mucus and a high concentration of ionic species in the planarian samples caused the current across the CE capillary to drop off, preventing samples from being analyzed.
A sample preparation method using a de-salting step with isopropyl alcohol and acetonitrile was developed that overcame this issue. With the addition of the de-salting step to sample preparation procedures, epinephrine was measured in an asexual and sexual tail segment as well as a sexual whole planarian worm. Current work is focused on building a library of monoamine neurotransmitters found in the head, trunk, and tail of sexual and asexual planarians.
Future work will involve characterizing aadc RNAi planarians and comparing their neurotransmitter profile with control animals and determine the role of monoamine neurotransmitters in germ cell development. Future work also involves interfacing a microfluidic ZipChip device with our mass spectrometers and exploring the ability of the ZipChip device to analyze planarian samples and increase the throughput of our current CE-MS system.

