 Ryan Miller joined the program as a second-year student in Chemical & Biomolecular Engineering in January 2020 and is expected to graduate in 2023. He completed his undergraduate degree in Chemical and Biochemical Engineering at Georgia Tech. His current research explores the microfluidic assembly of colloidal antioxidant crystal systems sensitive to intracellular oxidative stress. These drug delivery systems are being employed to tackle a range of diseases and injuries such as silver-ion induced cardiac tissue injury, alkali corneal injury, adipose-derived mesenchymal stem cell (adMSC) senescence, and reactive oxygen species (ROS) driven neurological disorders.
Ryan Miller joined the program as a second-year student in Chemical & Biomolecular Engineering in January 2020 and is expected to graduate in 2023. He completed his undergraduate degree in Chemical and Biochemical Engineering at Georgia Tech. His current research explores the microfluidic assembly of colloidal antioxidant crystal systems sensitive to intracellular oxidative stress. These drug delivery systems are being employed to tackle a range of diseases and injuries such as silver-ion induced cardiac tissue injury, alkali corneal injury, adipose-derived mesenchymal stem cell (adMSC) senescence, and reactive oxygen species (ROS) driven neurological disorders.
In November of 2020, Ryan presented his research titled “Stimulus-responsive antioxidant drug crystals and their health and ecological implications” at the Annual American Institute of Chemical Engineers (AIChE) Conference in Boston, MA. Additionally, he has presented his works at the 2020 and 2021 MBM Retreats.
His first-author publication has been recently submitted to ACS Applied Materials and Interfaces under the title “Extending the bioavailability of hydrophilic antioxidants for metal ion detoxification via recrystallization with polysaccharide dopamine”. Miller was also a co-author on the article “Histatin-1 is an endogenous ligand of the sigma-2 receptor” published in the FEBS Journal and has a patent pending pertaining to ROS-responsive polymers used for antioxidant crystal delivery.
Currently, he is completing lab rotations under the advisement of Hyunjoon Kong and Hee-Sun Han. He began a collaboration with Young Jun Kim’s lab at KIST-Europe aimed at nullifying the toxic effects of metal ions and endocrine disrupting factors such as bisphenol A on aquatic health in 2019. Miller also has an ongoing collaboration with Dr. Vinay Aakalu at UIC pertaining to treatment of alkali corneal injury.
Miller has participated in the Special Topics in MBM course and other trainee activities such as the Summer Journal Club readings/discussion meetings and Frontiers in Miniature Brain Machinery lectures. He served on the MBM Student Leadership Council (2020-21) and helped coordinate the 2021 Summer Journal Club. He also introduced Noo Li Jeon for his March 17, 2021 Frontiers in MBM lecture, as well as Franklin Carrero-Martinez’s plenary lecture at the 2021 MBM Retreat.
His community outreach activities include mentorship of two undergraduate and two high school students part of the Emergent Behaviors of Integrated Cellular Systems (EBICS) and the SParking High schoolers’ Excitement for Research in Engineering and Science (SpHERES) programs during the summers of 2020 and 2021. He has completed coursework in integrative neuroscience in addition to his coursework in chemical and biomolecular engineering.
Research Highlights (in his own words):
Reactive oxygen species (ROS) are inevitable by-products of cellular respiration, and when maintained at normal physiological levels, mediate intracellular signaling responsible for the regulation of cell adhesion, proliferation, differentiation, senescence, and apoptosis.[1–5] Furthermore, balanced ROS levels are necessary for proper stimulation of tissue-forming cells and immune cells responsible for enhanced wound healing.[5-9] However, the overproduction of ROS commonly caused by proinflammatory cytokine signaling, pathogenic bacterial infections, dysfunction of the mitochondria, and suppression of antioxidant signaling can be problematic for normal cellular function.[10–13] To maintain a physiological balance of ROS, cells have a sophisticated antioxidant regulatory system; however, disruption in homeostasis can overwhelm endogenous antioxidant defense and result in injury and disease pathogenesis.
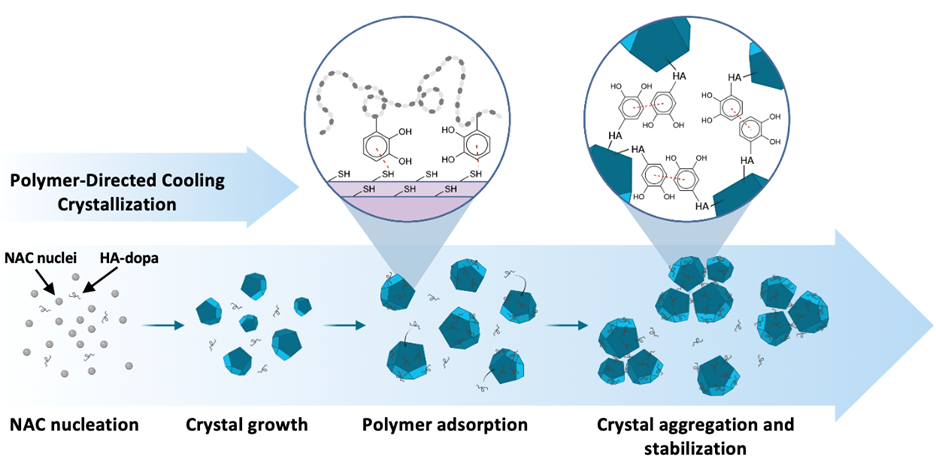
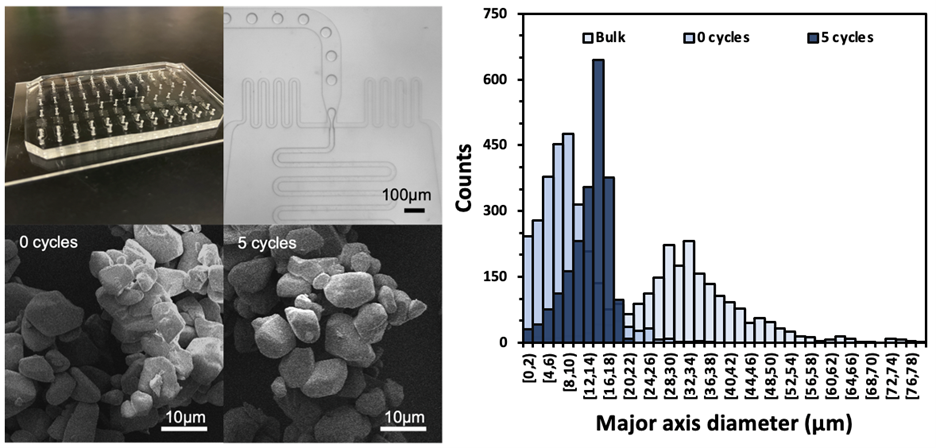
As such, my research explores new manufacturing and antioxidant delivery approaches to control a cell’s oxidative environment with the aim to improve the therapeutic efficacy of treatment of ROS-mediated injuries and diseases such as cardiac inflammation, corneal burns, and epilepsy. Through polymer-directed assembly of N-acetylcysteine colloidal crystals (Figure 1) we have been able to illustrate improved drug stability and ROS-dependent release. Control of antioxidant release profiles dependent on the stage of injury allows us to maintain ROS homeostasis, and drastically improve wound healing. Furthermore, we have transitioned from conventional batch crystallization to microfluidic assembly of these crystals in highly-monodisperse drops that act as individual reactors (Figure 2). We have also coupled a thermal cycling approach that can allow for precise crystal size and crystal size distribution control necessary for targeted drug delivery.
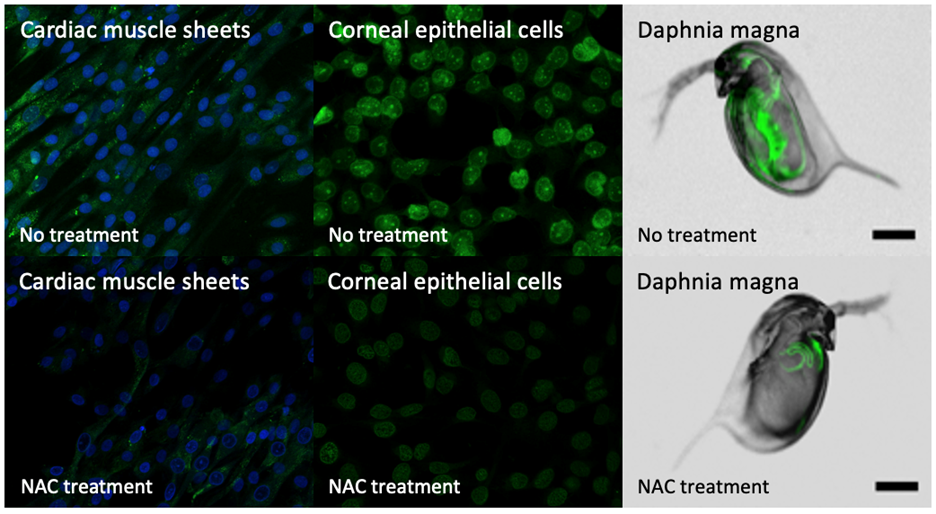
We have explored multiple triggers for oxidative stress in cells such as silver-ions and alkali chemicals. Following induced intracellular oxidative stress, we’ve investigated the efficacy of our antioxidants crystals by looking at their ROS-scavenging potential in vitro and in vivo. Fluorescence microscopy was used to visualize intracellular oxidative stress with a dye that fluoresces green upon oxidation (Figure 3). We have been able to successfully show the ability to control intracellular oxidative stress levels in cardiac muscle sheets and daphnia magna exposed to silver-ions and corneal epithelial cells exposed to sodium hydroxide. Our current work continues to explore oxidative stress at the ocular surface, prevention of senescence propagation in adMSC culture, and ROS-driven pathogenesis of neurological disorders.
Citations:
[1] D’Autréaux, B.; Toledano, M. B. ROS as Signalling Molecules: Mechanisms That Generate Specificity in ROS Homeostasis. Nature Reviews Molecular Cell Biology. October 2007, pp 813–824. https://doi.org/10.1038/nrm2256.
[2] Chiarugi, P.; Pani, G.; Giannoni, E.; Taddei, L.; Colavitti, R.; Raugei, G.; Symons, M.; Borrello, S.; Galeotti, T.; Ramponi, G. Reactive Oxygen Species as Essential Mediators of Cell Adhesion: The Oxidative Inhibition of a FAK Tyrosine Phosphatase Is Required for Cell Adhesion. Journal of Cell Biology 2003, 161 (5), 933–944. https://doi.org/10.1083/jcb.200211118. [15] Schieber, M.; Chandel, N. S. ROS Function in Redox Signaling and Oxidative Stress. Current Biology. Cell Press May 19, 2014. https://doi.org/10.1016/j.cub.2014.03.034.
[3] Kim, B. S.; Leong, J.; Yu, S. J.; Cho, Y.; Park, C. G.; Kim, D. H.; Ko, E.; Im, S. G.; Lee, J.; Kim, Y. J.; Kong, H. Stimulus-Responsive Anti-Oxidizing Drug Crystals and Their Ecological Implication. Small 2019, 15 (21). https://doi.org/10.1002/smll.201900765.
[4] Redza-Dutordoir, M.; Averill-Bates, D. A. Activation of Apoptosis Signalling Pathways by Reactive Oxygen Species. Biochimica et Biophysica Acta – Molecular Cell Research. Elsevier B.V. December 1, 2016, pp 2977–2992. https://doi.org/10.1016/j.bbamcr.2016.09.012.
[5] Morimoto, H.; Iwata, K.; Ogonuki, N.; Inoue, K.; Atsuo, O.; Kanatsu-Shinohara, M.; Morimoto, T.; Yabe-Nishimura, C.; Shinohara, T. ROS Are Required for Mouse Spermatogonial Stem Cell Self-Renewal. Cell Stem Cell 2013, 12 (6), 774–786. https://doi.org/10.1016/j.stem.2013.04.001.
[6] Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N. T. Reactive Oxygen Species (ROS) and Wound Healing: The Functional Role of ROS and Emerging ROS-Modulating Technologies for Augmentation of the Healing Process. International Wound Journal 2017, 14 (1), 89–96. https://doi.org/10.1111/iwj.12557.
[7] Trachootham, D.; Lu, W.; Ogasawara, M. A.; Valle, N. R. del; Huang, P. Redox Regulation of Cell Survival. Antioxidants and Redox Signaling. August 1, 2008, pp 1343–1374. https://doi.org/10.1089/ars.2007.1957.
[8] Reactive Oxygen Species in Cell Fate Decisions, Essentials of Apoptosis: A Guide for Basic and Clinical Research. Humana Press: Totowa, NJ, 2009; p 199-221
[9] Soneja, A.; Drews, M.; Malinski, T. Role of Nitric Oxide, Nitroxidative and Oxidative Stress in Wound Healing; 2005.
[10] Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. International Journal of Molecular Sciences. MDPI AG May 2, 2019. https://doi.org/10.3390/ijms20102407.
[11] Turrens, J. F. Mitochondrial Formation of Reactive Oxygen Species. Journal of Physiology. October 15, 2003, pp 335–344. https://doi.org/10.1113/jphysiol.2003.049478.
[12] Lin, P. W.; Myers, L. E. S.; Ray, L.; Song, S. C.; Nasr, T. R.; Berardinelli, A. J.; Kundu, K.; Murthy, N.; Hansen, J. M.; Neish, A. S. Lactobacillus Rhamnosus Blocks Inflammatory Signaling in Vivo via Reactive Oxygen Species Generation. Free Radical Biology and Medicine 2009, 47 (8), 1205–1211. https://doi.org/10.1016/j.freeradbiomed.2009.07.033.
[13] Hensley, K.; Robinson, K. A.; Gabbita, S. P.; Salsman, S.; Floyd, R. A. Forum: Therapeutic Applications of Reactive Oxygen and Nitrogen Species in Human Disease REACTIVE OXYGEN SPECIES, CELL SIGNALING, AND CELL INJURY; 2000.

