 As one of our initial trainees, Brian Baculis joined the program as a second-year graduate student January 1, 2018 and is expected to graduate in 2021. His major is neuroscience and he completed his undergraduate degree in neuroscience at Temple University. His research topics are learning, memory, and plasticity; neural plasticity; and psychiatric and neurological illness. His research techniques include biochemistry, electrophysiology, immunohistochemistry, and imaging. Some of his recent projects include G-protein inward rectifying potassium channel dysregulation upon seizure activity, regulation of the Kv7 channel complex at the axonal initial segment of hippocampal neurons during homeostatic scaling of intrinsic excitability, abnormal neuronal signaling in Alzheimer’s disease, and mitochondria transport and function dysregulation in epilepsy.
As one of our initial trainees, Brian Baculis joined the program as a second-year graduate student January 1, 2018 and is expected to graduate in 2021. His major is neuroscience and he completed his undergraduate degree in neuroscience at Temple University. His research topics are learning, memory, and plasticity; neural plasticity; and psychiatric and neurological illness. His research techniques include biochemistry, electrophysiology, immunohistochemistry, and imaging. Some of his recent projects include G-protein inward rectifying potassium channel dysregulation upon seizure activity, regulation of the Kv7 channel complex at the axonal initial segment of hippocampal neurons during homeostatic scaling of intrinsic excitability, abnormal neuronal signaling in Alzheimer’s disease, and mitochondria transport and function dysregulation in epilepsy.
In October 2018, he presented his research on “Regulation of Kv7 channel complex at the axonal initial segment of hippocampal neurons during homeostatic scaling of intrinsic excitability” at the MBM Retreat. In 2019 his research into “Counting growth factors in single cells with infrared quantum dots to measure discrete stimulation distributions” was published in Nature Communications. In September 2019, his lightning talk on “Homeostatic regulation of ion channels at the axon initial segment” at the MBM Retreat received Honorable Mention. He presented his research on “Regulation of Kv7 and Nav channels at the axon initial segment of hippocampal neurons during homeostatic scaling of intrinsic excitability” at the 2019 Society for Neuroscience Conference and submitted a mini-review of “The Role of Kv7 Channels in neural Plasticity and behavior” to Frontiers Kv7 channels: structure, Physiology and pharmacology in 2020. He has completed lab rotations under the advisement of Hee Jung Chung and Gabriel Popescu.

Baculis has completed five semesters of the Special Topics in MBM course and participated in other trainee activities such as the Kickoff meeting, Summer Journal Club readings/discussion meetings, Frontiers in Miniature Brain Machinery lectures, and the 2018, 2019, & 2020 MBM Retreats. He served on the MBM Student Leadership Council from 2019 to 2020. His community outreach activities through MBM include assisting with the MBM booths at the Beckman Open House in March 2019 and the Urbana Farmer’s Market in June 2019. He participated in public outreach through the Neuroscience Department’s Student Village SfN Night and as Spring Lunch Coordinator for Seminar Help in the spring of 2018. In addition, he has mentored three undergraduate students through the Hee Jung Chung Lab and one undergraduate through the Gabi Popescu lab.
Baculis has completed additional coursework in advanced topics in neuroscience, brain behavior & info process, cell and molecular neuroscience, integrative neuroscience, and research ethics and responsibilities. He attended the Science Communication Workshop led by Sandra Tsing Loh at Beckman Institute for Advanced Science and Technology April 17-19, 2018, as well as the Alda Communication Science Lecture September 20, 2018. In August 2018, Baculis participated in the EBICS Engineering Cellular Systems Summer School. In November 2019, he attended workshops on Transforming Conflict into Collaboration and Responsible Conduct of Research.
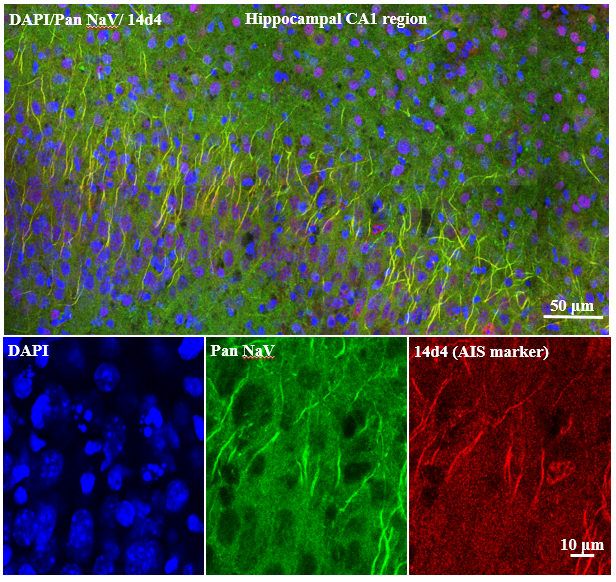
Voltage-Gated Sodium Channels at the Axonal Initial Segment: Imaged at the Core Facilities at the Carl R. Woese Institute for Genomic Biology by Brian Baculis.Research Highlights (in his own words):
The goal of my research is to understand the molecular mechanisms and signaling cascades that underlie homeostatic regulation of intrinsic excitability. In vitro studies using dissociated hippocampal culture and organotypic slice culture have revealed scaffolding protein (ankyrin-G) and voltage-gated sodium channels are regulated by prolonged activity blockade. Using spatial light interference microscopy (SLIM) imaging, I plan to show real-time imaging of the change in the localization of the axon initial segment after prolonged activity blockade. One potential kinase that may be involved in this process is Casein kinase 2 (CK2) and in order to test this hypothesis I have begun to test CK2 inhibitors (TBCA) and (TBB) and utilizing an overexpression of wild-type CK2 and kinase in-active CK2 (K68M) in dissociated culture to test if a decrease in CK2 activity is responsible for scaling intrinsic excitability and voltage-gated sodium and potassium channel localization at the axon initial segment. In addition, I have begun to investigate the role of MAPK/ERK pathway on homeostatic regulation of intrinsic excitability. Using an in vivo model of prolonged activity blockade, I plan show similar changes of homeostatic scaling intrinsic excitability and voltage-gated sodium and potassium channel localization at the axon initial segment.

